Greening Soda Ash: Production and Protection
Soda ash is an important chemical raw material with extensive applications and massive global annual production. Driven by current environmental protection policies, many industries have embarked on paths toward green transformation, and soda ash production is no exception. Furthermore, soda ash itself contributes to environmental protection efforts. How can the soda ash production process achieve green transformation? What role can soda ash play in supporting the environmental protection industry? This article will explore these two questions in depth.
1. Greening Soda Ash Production
Technological upgrades in the soda ash production process can reduce its energy consumption, emissions, and pollution. This reduction in environmental impact, achieved through internal innovation, is a core part of its green transformation.
1.1 Process Route Optimization of Soda Ash
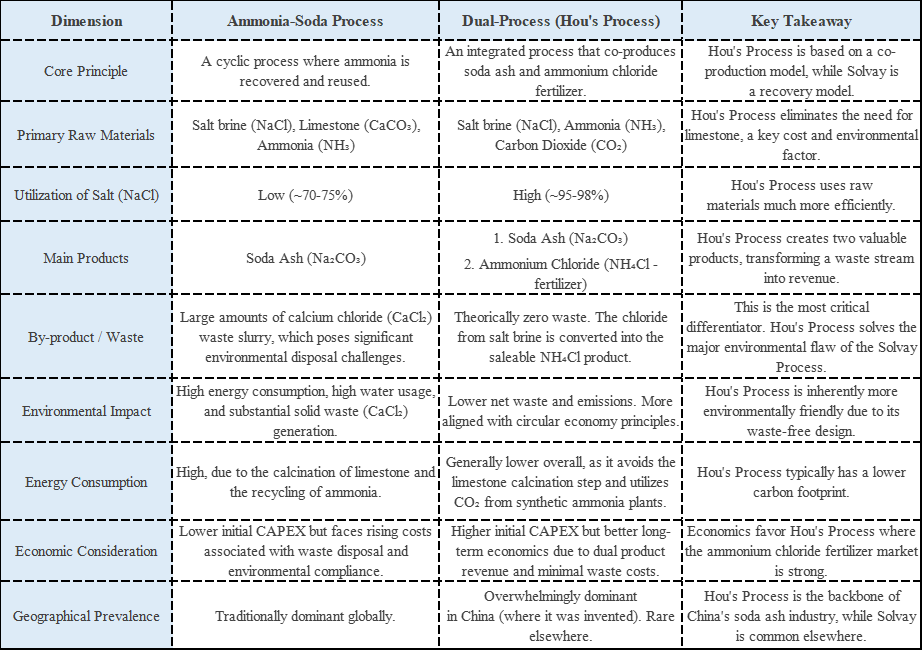
The ammonia-soda process, developed by Chinese chemist Hou Debang, revolutionized sodium carbonate production. By integrating the ammonia-soda method with the synthetic ammonia industry and using carbon dioxide to treat reaction products, this method significantly reduces the generation of calcium chloride waste and minimizes pollution. Furthermore, the process achieves a high raw material utilization rate, making near-total use of sodium and chloride ions from salt to produce both sodium carbonate and ammonium chloride fertilizer, thereby improving cost-effectiveness. The highly integrated production workflow also reduces energy loss throughout the process.
1.2 Applications for Energy Efficiency
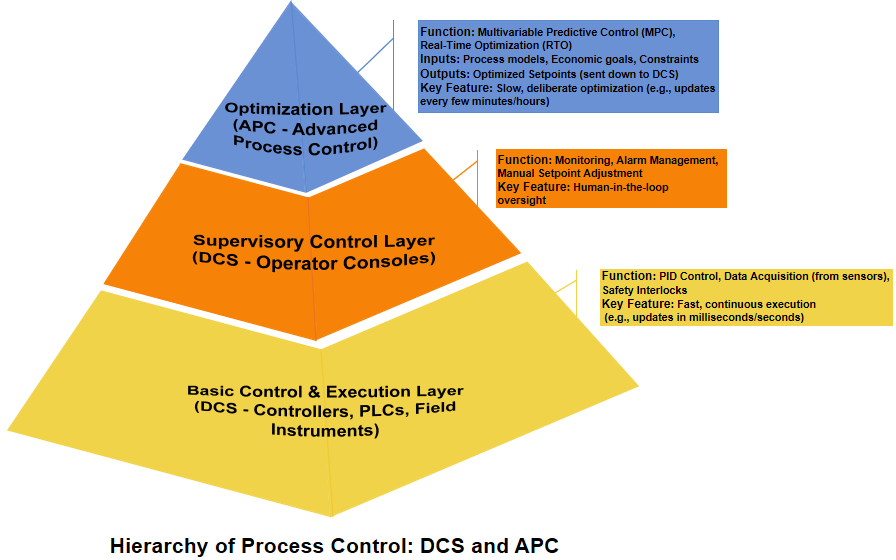
To significantly enhance energy efficiency, washing soda plants implement a comprehensive strategy across three key areas. First, through heat recovery, factories harness waste heat following the principle of ‘cascading utilization and temperature matching,’ using high-grade heat for power generation and medium-to-low-grade heat for heating and cooling, drastically cutting overall energy consumption and costs. Second, via equipment upgrades, the adoption of high-efficiency, large-scale machinery like large carbonization towers and steam calcination furnaces boosts productivity while reducing energy use per unit output. Finally, with process automation, advanced Distributed Control Systems (DCS) and Advanced Process Control (APC) optimize process parameters to ensure production consistently runs at optimal levels, thereby minimizing energy waste.
1.3 From Waste to Resources: Emission Control
Carbon Capture and Utilization (CCU)
Washing soda production is a major source of carbon dioxide emissions, primarily from limestone calcination. Carbon Capture, Utilization and Storage (CCUS) technology presents a solution by capturing this CO₂ from industrial flue gas or directly from the atmosphere—though the latter remains more challenging and costly—and converting it into valuable products. The process consists of three core stages: capture, transport (via pipeline, ship, or vehicle), and utilization. At its core, CCUS transforms CO₂ into a resource, producing chemicals and fuels like methanol and plastics, construction materials, and commercial products for food processing and refrigeration. It is also used for enhanced oil recovery (EOR) by injection into oil fields.
By not only reducing emissions but also creating new business opportunities, CCUS transforms CO₂ from a waste product into a valuable resource, establishing a circular economy model and serving as a key technological pathway toward achieving carbon neutrality.
Wastewater Treatment
The traditional ammonia-soda process produces large volumes of wastewater, a challenge addressed by a philosophy of resource recovery and recycling. Process water is reused following the ‘graded use and cyclic application’ principle to minimize consumption and discharge. For wastewater that must be released, ammonia is recovered via steam stripping. In this process, wastewater flowing down a stripping tower encounters steam flowing up, which volatilizes ammonia (NH₃-N) for recapture and reuse. The stripped wastewater, now low in ammonia but high in dissolved salts (CaCl₂ and NaCl), proceeds to desalination through multi-effect evaporation and fractional crystallization. Here, controlled heating concentrates the wastewater, causing salts to crystallize out sequentially. Recovered NaCl is recycled to the brine pool, while CaCl₂ is sold as a by-product. The condensed vapor is reused, and any final residue is landfilled, achieving comprehensive resource recovery and near-zero discharge.
Waste Residue Treatment
The resource utilization of waste slag is the mainstream approach in washing soda production, focusing on transforming waste into valuable resources. Key applications include: building material production, where white mud substitutes for limestone in cement, reducing both resource consumption and carbon emissions, or is mixed with alkali residue and other solid wastes to produce bricks; soil improvement, by neutralizing acidic farmland with calcium-rich residues; chemical recovery, to extract purified calcium carbonate or magnesium compounds for industrial use; environmental governance, such as using white mud (CaCO₃) as a flue gas desulfurizer to produce gypsum; and agricultural salt production from alkali residue via washing and separation.
2. Soda Ash in Environmental Protection
Disodium carbonate not only is produced through an increasingly green process but also plays a significant role in environmental protection. As an “environmental assistant,” it has a wide range of applications, which is a key aspect of its value beyond production.
2.1Flue Gas Desulfurization
Disodium carbonate is a major desulfurization agent for treating flue gas from power plants, steel mills, and waste incinerators. It effectively neutralizes sulfur dioxide (SO₂)—a key contributor to acid rain—through a simple acid-base reaction. Compared to lime, disodium carbonate reacts faster and achieves over 95% efficiency, while avoiding scaling issues and reducing maintenance.
The core reaction, Na₂CO₃ + SO₂ → Na₂SO₃ + CO₂↑, produces sodium sulfite. This can be intentionally oxidized into valuable sodium sulfate (Na₂SO₄, or Glauber’s salt) by injecting air: 2Na₂SO₃ + O₂ → 2Na₂SO₄.
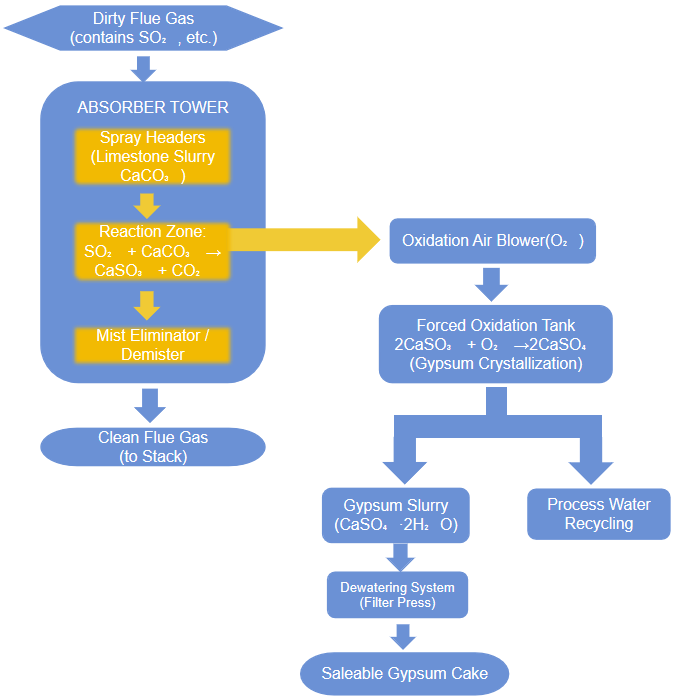
Thus, this process transforms a harmful pollutant into a marketable byproduct used in detergents, glass, textiles, and pulp. This resource recovery not only ensures emissions compliance but also offsets operating costs, making it a mature and efficient dry/semi-dry desulfurization technology.
2.2Water Treatment
Carbonic acid disodium salt is an excellent pH regulator and neutralizing agent. It is widely used in industrial and municipal wastewater treatment to neutralize acidic water, restoring it to a neutral pH to meet discharge standards. Furthermore, carbonic acid disodium salt reacts with calcium and magnesium ions in water, precipitating them as carbonates. This process reduces water hardness, softens water, and prevents scale formation in boilers and pipelines, thereby improving thermal efficiency and saving energy. It also aids in removing heavy metals: under alkaline conditions created by carbonic acid disodium salt, ions such as lead, copper, and zinc form insoluble hydroxide precipitates that can be removed through filtration.
2.3 Soil Remediation
Industrial soda plays a valuable role in soil management by addressing both acidity and contamination. In agriculture, it can be applied to neutralize acidic soils caused by the overuse of chemical fertilizers, thereby improving soil structure and restoring fertility. Furthermore, in the remediation of soils polluted with heavy metals, industrial soda alters the chemical environment to transform these metals into less soluble forms. This process significantly reduces their bioavailability and mobility, preventing them from being easily absorbed by plants.
3.Conclusion
The calcined soda industry’s green transformation is more than just regulatory compliance; it embodies a profound reinvention. This shift from a high-consumption model to a circular economy marks a bold blueprint for change. While achieving its own sustainability, the industry now also empowers others, becoming an environmental ‘contributor.’
Thus, its role has expanded far beyond a basic chemical supplier. It is not only essential to industries like glass and detergents but also a leader in green manufacturing, a model of circular economy practices, and a driver of carbon reduction. This transformation proves that traditional industry and environmental health are not opposites. Through innovation and systemic thinking, the calcined soda industry has blazed a trail for the entire traditional manufacturing sector, demonstrating that economic and ecological goals can align.