Unlocking the Secrets Behind Sodium Bicarbonate’s Versatility
In the vast world of industrial raw materials, Sodium Bicarbonate stands as a testament to unassuming power. Its simple formula, NaHCO₃, belies an incredible capacity to solve complex challenges from the food production line to the smokestack. For engineers, formulators, and procurement specialists, a pressing question remains: what are the fundamental secrets that unlock such wide-ranging versatility in a single compound?

The answer is not found in a single, magical trait, but in a powerful synergy of well-understood chemical behaviors and highly customizable physical characteristics. Decoding these properties is the key to optimizing your processes, enhancing your products, and driving innovation in your field.
The Four Pillars of Performance
The multifaceted nature of this versatile compound rests on four foundational pillars. Together, they create a toolbox of functionalities that can be precisely matched to specific industrial needs.
Secret 1: The Power of Gentle Alkalinity
Sodium hydrogen carbonate presents a mild alkaline character in solution (pH ~8.3 for a 1% solution). This precise level of basicity is one of its most strategically valuable assets.
The Scientific Basis: Unlike the aggressive and corrosive action of strong alkalis like sodium hydroxide, baking soda offers a gentle and controllable neutralizing power. It effectively neutralizes acids without causing significant damage to equipment, materials, or biological tissues, making it a safer choice for a multitude of applications.
Industrial Applications in Action: This “gentle strength” is why it is safely employed in food processing to adjust acidity, in personal care formulations for mild cleansing, and in cleaning sensitive machinery where harsh chemicals could cause damage. It solves problems effectively while minimizing unwanted side effects.

Secret 2: Mastery of pH Stability
Beyond simple neutralization, this common salt performs as an exceptional pH buffer, making it indispensable for processes requiring a stable chemical environment.
The Scientific Basis: In an aqueous solution, the substance establishes a bicarbonate (HCO₃⁻) buffer system. This system acts as an intelligent “acid-base sponge,” automatically absorbing excess hydrogen ions (H⁺) in acidic conditions or releasing them in basic conditions. This innate ability maintains a stable pH within a narrow, optimal range.
Industrial Applications in Action: This buffering capability is crucial in swimming pool and spa treatment to prevent corrosive pH swings. It ensures the stability of active ingredients in pharmaceuticals and cosmetics throughout their shelf life. Furthermore, it provides a consistent reaction environment in various industrial chemical processes, maximizing yield and product quality.

Secret 3: On-Demand Gas Generation
One of the most dynamic properties of NaHCO₃ is its ability to decompose predictably upon heating or when in contact with acids, instantly producing carbon dioxide (CO₂) gas.
The Scientific Basis: The decomposition follows a straightforward reaction pathway: NaHCO₃ + H⁺ → Na⁺ + H₂O + CO₂↑. This reaction is highly reliable, controllable, and leaves behind minimal, typically benign, residues.
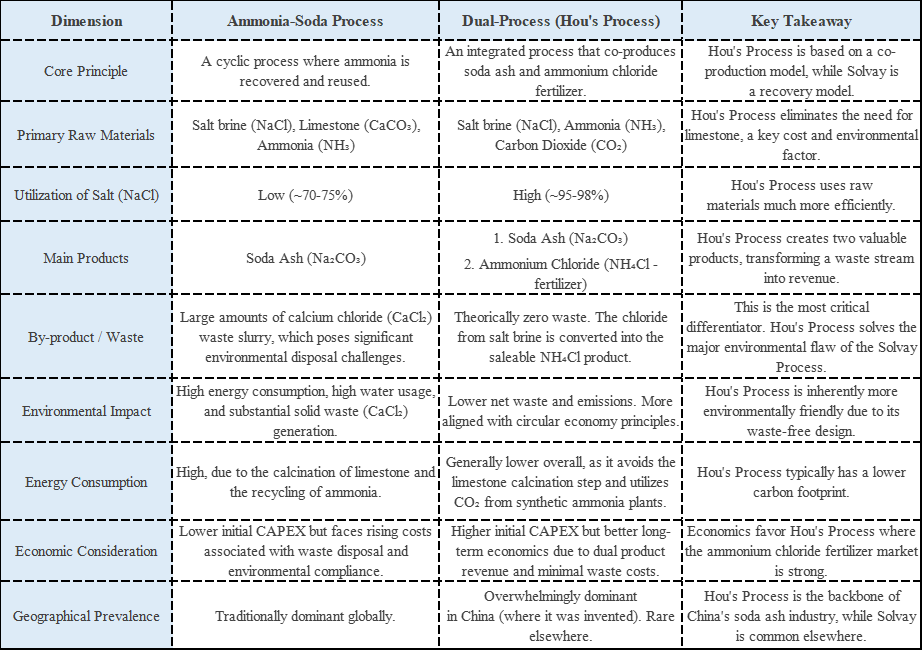
Industrial Applications in Action: This single property powers a stunning array of applications. It serves as a leavening agent in baking, making dough rise. It functions as a blowing agent in the polymer industry to create lightweight foam plastics. In fire extinguishers, the rapid CO₂ release smothers flames effectively. This is a prime example of one property enabling multiple performances.
Secret 4: Engineered Physical Form
The final secret to this agent’s success lies not just in its chemistry, but in the customizability of its physical form.
The Scientific Basis: The particle size, morphology, and density of the powder are not fixed. They can be precisely engineered. Finer powders provide a larger surface area for rapid reactions, while specific particle distributions ensure optimal flowability, dispersion, and suspension in various formulations.

Industrial Applications in Action: This physical engineering allows for application-specific optimization. For flue gas desulfurization, we provide ultra-fine “activated” grades that achieve millisecond-level reactions with acidic pollutants. For the baking industry, we engineer leaveners with perfect particle size profiles for controlled gas release. Precise morphology is what transforms a generic powder into a high-performance, tailored solution.
From Understanding to Application: Choosing the Right Solution
Understanding these four pillars is the first step. The critical next step is matching the right grade of Sodium Bicarbonate to your specific application. The requirements for a technical-grade product used in industrial cleaning are vastly different from those of a high-purity pharmaceutical-grade used in tablet formulations or a food-grade product in consumer goods.
The impurity profile, alongside the engineered physical properties, directly determines the success and safety of the application.
Your Partner in Performance
We are more than just a supplier of Sodium Bicarbonate. We are your technical partner in unlocking its full potential for your business. Our expertise lies in precisely controlling these chemical and physical properties to deliver stable, efficient, and professional solutions for your unique challenges.
Contact us today to speak with our technical experts. Let us help you decode the perfect Sodium Bicarbonate solution for your application, driving efficiency and innovation in your operations.