Sodium Bicarbonate: A Safer, More Cost-Effective Solution for Industrial pH Control
Sodium bicarbonate is redefining standards in industrial pH control, offering process engineers and plant managers a demonstrably safer and more economical alternative to traditional strong alkalis. Whether managing wastewater, chemical synthesis, or gas scrubbing, achieving precise and stable pH is critical. While caustic soda (NaOH) or lime may seem like the default for their aggressive reactivity and lower upfront cost, a deeper analysis reveals why this versatile alkali—essentially baking soda in its pure industrial form—is the smarter strategic choice for modern, efficient operations.
True process cost extends far beyond the price per metric ton. Selecting sodium bicarbonate as your primary buffering and neutralizing agent is an optimization grounded in Total Cost of Ownership (TCO). Its mild, controllable chemistry enables leading facilities worldwide to achieve safer, more stable, and ultimately lower-cost outcomes.

Beyond Basic Neutralization: The Technical Edge of Bicarbonate
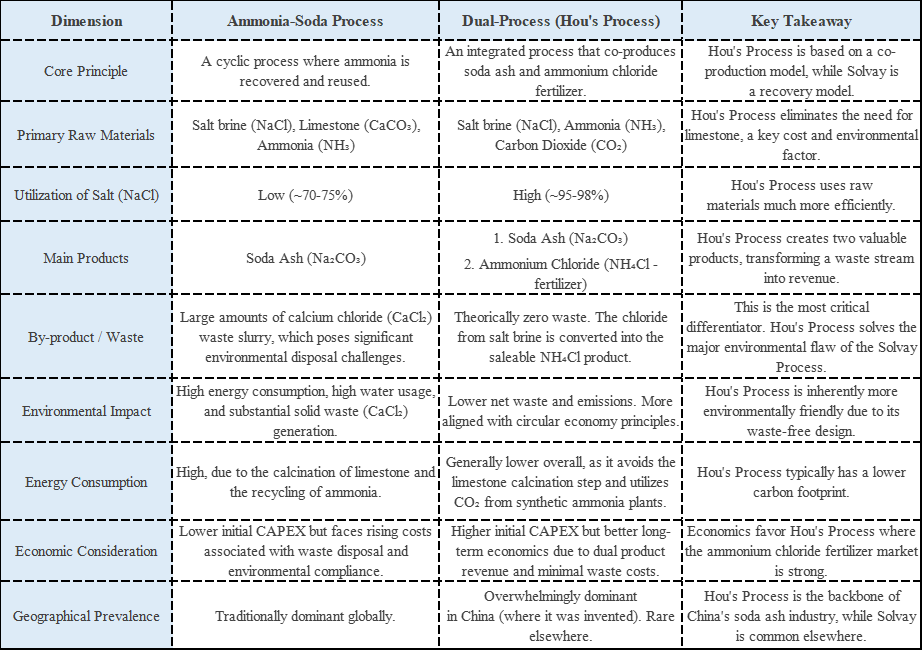
Unlike strong alkalis that cause a sharp, difficult-to-control pH spike, NaHCO₃ works differently. It establishes a natural carbonic acid/bicarbonate buffer system in water. This chemistry translates into tangible process advantages
Prevents Destructive pH Over-shoot
It stabilizes pH within a mild, ideal range (typically 8-9.5 when fully converted), eliminating the risk of a runaway reaction from accidental overdosing. This protects sensitive downstream biology in wastewater or prevents dissolved metals from re-precipitating.
Ensures a Controlled, Predictable Reaction
The release of alkalinity is gradual and self-limiting. This minimizes risks of local overheating or violent reactions, making it ideal for systems with volatile compounds or temperature-sensitive processes.
Inherently Safer to Handle
As a non-corrosive, non-hazardous solid, sodium hydrogen carbonate drastically reduces risks associated with storage, handling, and dosing. It diminishes the need for expensive corrosion-resistant equipment, stringent PPE, and extensive safety training.

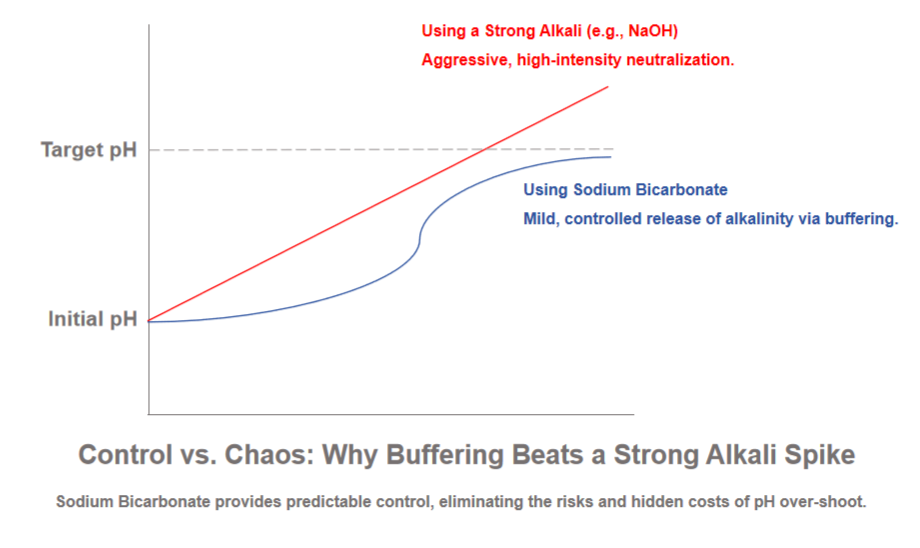
The Total Cost of Ownership (TCO) Analysis
A simplistic view focuses on unit chemical cost, where strong alkalis often appear cheaper. A comprehensive TCO model reveals a different story. Here’s a breakdown of key cost factors
The verdict is clear: While the upfront price of this buffering agent may be higher, the significant savings in safety management, operational simplicity, process stability, and waste disposal consistently deliver a lower Total Cost of Ownership.
Optimizing Your Process with Bicarbonate
To maximize the benefits of switching, consider these key implementation points
Implement Precise Dosing
Pair the compound with an automated dosing system controlled by an online pH probe. Its buffering action is perfectly suited for stable, closed-loop control, minimizing waste and labor.
Select the Right Particle Size
Choose a bicarbonate of soda grade that matches your reaction speed needs. Fine powders offer rapid dissolution for quick adjustments, while coarser granules provide a sustained, slow-release effect for continuous processes.

Ensure Proper Mixing
For large-scale applications, use a pre-dissolution tank or an efficient inline mixer to ensure complete solution homogeneity before it enters the main process stream, preventing uneven reactions.
Case Study: Real-World Savings in Electroplating
A precision electroplating facility was using sodium hydroxide to neutralize acidic rinse water containing nickel and copper. They faced erratic pH control, occasional effluent violations, and significant operator safety concerns.
After a full audit, they switched to sodium bicarbonate as the primary neutralizer, coupled with a simple automated feeder. The results were transformative:
pH control stability improved by over 60%, eliminating non-compliance events.
Operators could handle the pH adjustment process without full chemical suits, improving morale and productivity.
While their annual chemical spend increased by approximately 15%, the dramatic reductions in safety maintenance, labor hours, and compliance risk led to a net decrease of about 8% in total annual operating costs.

Conclusion: Partnering for Smarter Process Chemistry
Selecting a pH control agent is a strategic decision that impacts safety, compliance, efficiency, and profitability. Sodium bicarbonate offers a demonstrably smarter, safer, and more sustainable path forward.
As a specialized producer, we provide more than just consistent, high-quality product. Our technical support team partners with you to analyze your specific application, from initial process optimization trials to full-scale implementation support.
Ready to see if this versatile alkali is the right solution for your operation? Contact our application engineering team today for a confidential, professional consultation and a tailored evaluation of your pH control process.