Soda Ash vs. Baking Soda: The Essential Guide
In chemical production and procurement, few materials are as frequently confused as soda ash and baking soda. Despite their similar names and shared sodium content, these two compounds serve distinctly different purposes across industries. Understanding their differences isn’t just academic—it’s crucial for operational safety, product quality, and cost efficiency.
This comprehensive guide breaks down the essential differences between soda ash (sodium carbonate) and baking soda (sodium bicarbonate), providing you with the knowledge to make informed decisions for your specific applications.
1. Chemical Properties: The Molecular Divide
At the molecular level, these compounds reveal their fundamental differences
Soda Ash (Sodium Carbonate, Na₂CO₃)
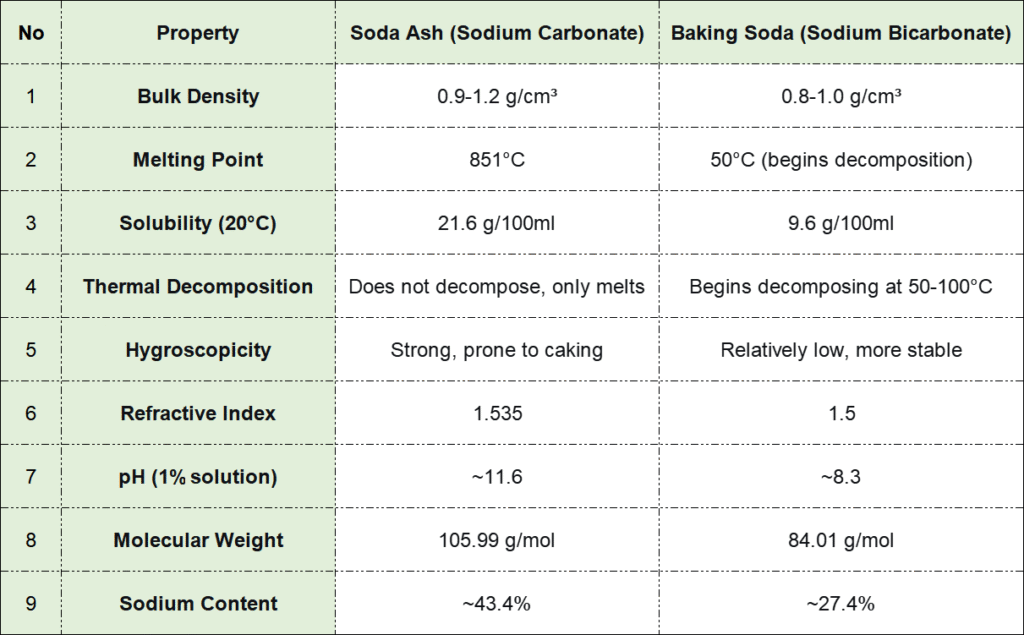
Molecular weight: 105.99 g/mol
Sodium content: ~43.4%
Chemical classification: Salt of strong base (sodium hydroxide) and weak acid (carbonic acid)
Hydrolysis reaction: Na₂CO₃ + H₂O → 2Na⁺ + HCO₃⁻ + OH⁻
Baking Soda (Sodium Bicarbonate, NaHCO₃)
Molecular weight: 84.01 g/mol
Sodium content: ~27.4%
Chemical classification: Acidic salt
Hydrolysis reaction: NaHCO₃ + H₂O → Na⁺ + H₂CO₃ + OH⁻
The pH difference is particularly significant. A 1% sodium carbonate solution reaches pH 11.6 (strongly alkaline), while a 1% sodium bicarbonate solution maintains pH 8.3 (weakly alkaline). This variance explains baking soda’s superior buffering capacity and milder chemical behavior.


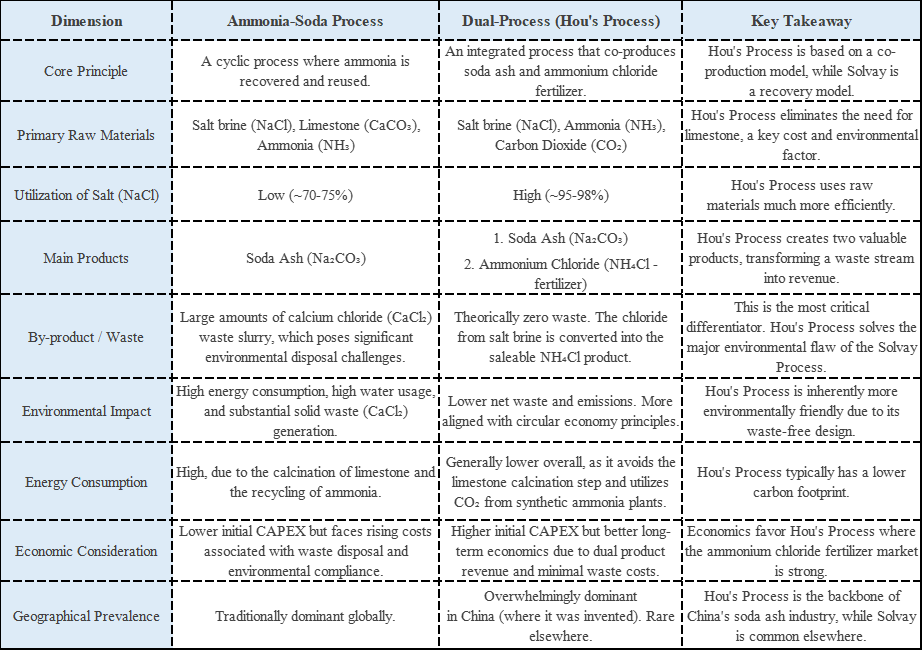
2. Production Processes: Different Paths to Creation
The manufacturing processes further highlight their distinctions
Soda Ash Production
Ammonia-soda (Solvay) process: Dominant industrial method using sodium chloride, ammonia, and carbon dioxide
Hou’s process: Chinese-developed method improving material utilization
Natural mining: Extracting and refining trona ore
Sodium Bicarbonate Production
Carbonation method: Introducing CO₂ into purified soda ash solution
Double decomposition: Reacting sodium salts with ammonium bicarbonate
3. Physical Properties: A Side-by-Side Comparison
4. Thermal Behavior: Stability vs. Decomposition
Thermal properties reveal crucial functional differences:
Soda ash demonstrates remarkable thermal stability, melting at 851°C without decomposition. Upon cooling, it forms a glassy solid (sodium carbonate glass).
Baking soda begins decomposing at temperatures as low as 50°C, with rapid decomposition occurring between 100-150°C:
2NaHCO₃ → Na₂CO₃ + H₂O + CO₂↑
This low-temperature decomposition makes baking soda invaluable for applications requiring gas generation, such as baking and fire suppression.
5. Professional Identification Methods
Laboratory Techniques
Phenolphthalein test: Soda ash turns deep red; baking soda shows faint pink
HCl reaction: Baking soda effervesces violently; soda ash reacts mildly
Thermal test: Baking soda shows ~36.9% mass loss; soda ash shows none
Field Techniques
Texture test: Soda ash feels slippery; baking soda feels gritty
Dissolution test: Soda ash dissolution is exothermic (warms container); baking soda is endothermic (cools container)
pH paper: Soda ash turns paper dark blue/purple; baking soda shows light blue/green
6. Storage & Handling: Safety First
Soda Ash Requirements
Store sealed in cool, dry, ventilated areas
Maintain relative humidity below 50%
Wear PPE (gloves, eye protection) due to its corrosive nature
Keep away from acids
Baking soda Requirements
Store sealed at room temperature
Avoid high temperatures to prevent decomposition
Generally safe with minimal protective equipment needed
7. Application-Based Selection Guide
Choose Soda Ash For
Strong alkalinity needs (industrial cleaning, pH adjustment)
High-temperature processes (glass manufacturing)
Water softening and treatment
Choose Baking Soda For
pH buffering (pool maintenance, medicinal uses)
Mild alkalinity (food processing, cosmetics)
Gas generation (baking, fire extinguishers)
Odor absorption and mild cleaning
8. Quality Assessment Parameters
Soda Ash Quality
High sodium carbonate content (>99%)
Low salt (NaCl) content
Low iron content
Baking Soda Quality
High sodium bicarbonate content
Appropriate pH (∼8.3 in solution)
Low moisture loss
Conclusion: Making the Right Choice
While soda ash and baking soda share some chemical relationships, their differences define their applications. Soda ash provides strong alkalinity and thermal stability, while baking soda offers mild alkalinity, buffering capacity, and gas generation properties.
Understanding these distinctions ensures not only optimal results in your processes but also enhances safety and cost-effectiveness. Whether you’re manufacturing glass, baking bread, treating water, or formulating chemicals, choosing the right sodium compound is crucial to your success.
For specific technical specifications or personalized guidance in selecting the right chemical for your application, contact our technical team for expert assistance and competitive quotations.