The Alkali and Hou Routes for Soda Ash: A Technical Comparison
Soda ash, an essential chemical raw material, has a massive annual global output and is indispensable to industries like glass, chemicals, and detergents. While naturally occurring trona ore serves as a traditional source, the two primary industrial production methods are the traditional ammonia-soda process and the innovative dual-process. This article provides an in-depth comparison of these two processes, analyzing their advantages and drawbacks from technological, economic, and environmental perspectives.

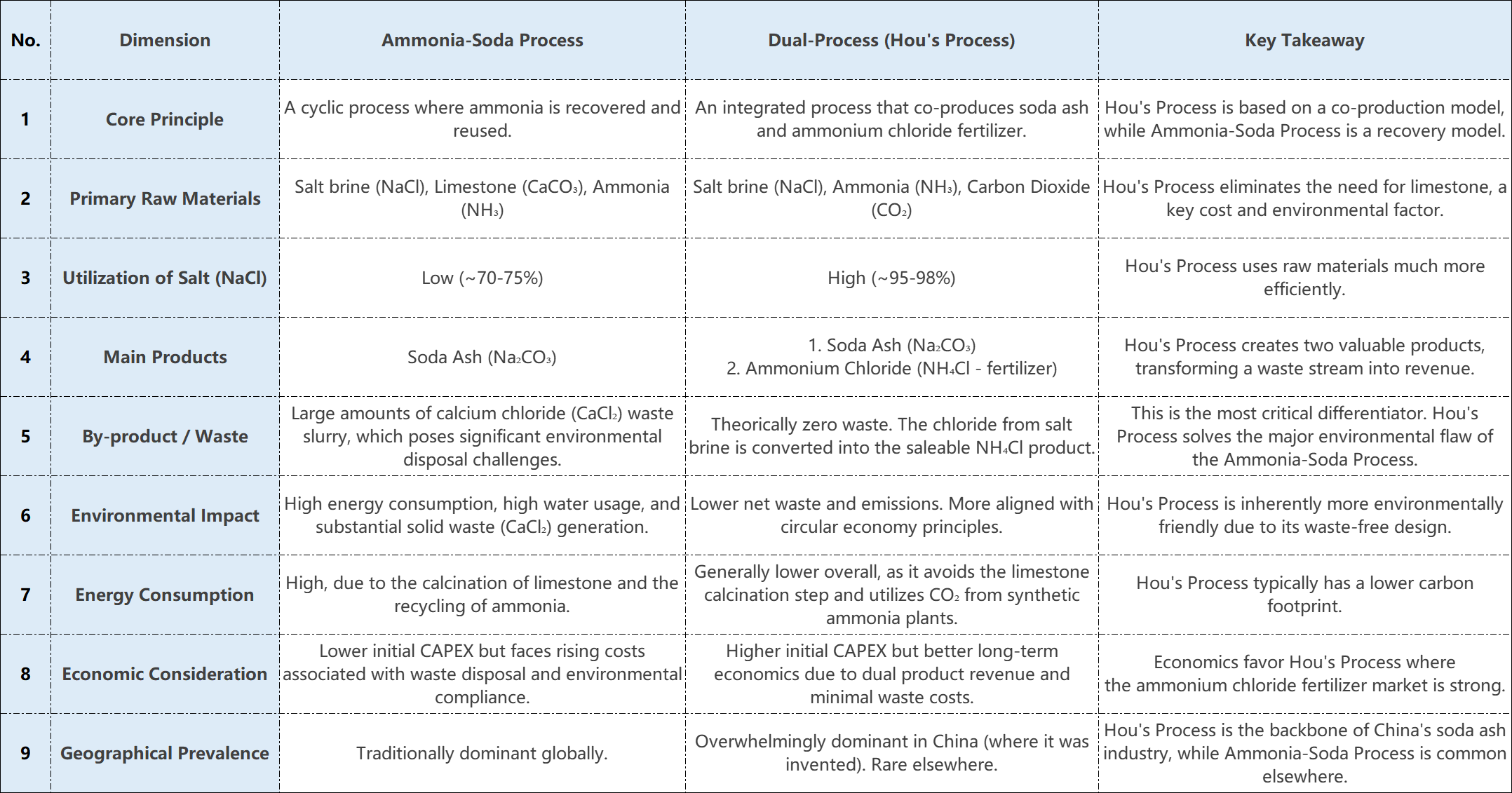
1.Brief Process Overview
The Alkali Method
In the ammonia-soda process, brine, limestone, and ammonia are used as core raw materials to produce sodium carbonate. Its key reaction, NaCl + NH₃ + CO₂ + H₂O → NaHCO₃ + NH₄Cl, yields sodium bicarbonate, which is subsequently calcined into sodium carbonate. While ammonia is recycled as a catalyst, the process generates substantial calcium chloride (CaCl₂) waste stream, a significant environmental and economic disadvantage that must be considered.
Hou’s Process( Dual-process)
An innovative improvement on the ammonia-soda process, the combined alkali process integrates directly with synthetic ammonia production. While its core reaction is identical to the initial stage of the ammonia-soda process, its subsequent processing is fundamentally different. It utilizes CO₂ byproduct from synthetic ammonia plants to co-produce disodium carbonate and ammonium chloride fertilizer. A key advantage of this integrated approach is its near-complete material utilization and minimal waste generation.
2.Core Comparison Dimensions
A comprehensive comparison shows that while the traditional ammonia-soda process has some inherent advantages, the combined alkali method is superior in both economic and environmental benefits.
Environmental Edge
The ammonia-soda process, as a traditional method, has a fatal flaw: the waste issue. This process requires limestone (CaCO₃) to decompose for producing carbon dioxide (CO₂) during ammonia (NH₃) recovery, resulting in a byproduct—calcium chloride (CaCl₂)—which is disposed of as waste liquid. Approximately 10 tons of calcium chloride-containing waste liquid is generated for every ton of washing soda (Na₂CO₃) produced. This waste accumulates in large quantities, occupying land, causing environmental pollution, and proving extremely difficult and costly to treat.
In contrast, the dual-alkali process eliminates the use of limestone for ammonia recovery, thereby avoiding the generation of calcium chloride altogether. Instead of externally treating ammonium chloride (NH₄Cl), this method promotes its crystallization from the mother liquor through cooling and salt (NaCl) addition. After separation and drying, it is converted into nitrogen fertilizer (ammonium chloride). Thus, chloride (Cl⁻) and ammonium (NH₄⁺) ions—which would have been discharged as waste in the ammonia-soda process—are transformed into marketable products in the dual-alkali process.
This approach not only completely resolves the environmental pollution issue but also greatly enhances raw material utilization, improves economic efficiency, and serves as a exemplary model of a “circular economy” in the chemical industry.
Economics
For China, the dual-alkali process (also known as the Hou’s process) serves as a “tailored” solution:The salt fields along the country’s eastern coast primarily produce sea salt. Compared to the extensive underground rock salt deposits found in the West, sea salt production entails higher costs and yields a lower concentration of sodium chloride. The dual-alkali process enables the simultaneous production of carbonate acid disodium salt and ammonium chloride, with utilization rates of sodium (Na⁺) and chloride (Cl⁻) exceeding 95%, thereby significantly conserving valuable salt resources.
As a major agricultural country, China has substantial demand for nitrogen fertilizers. The ammonium chloride (NH₄Cl) co-produced by this process serves as an effective nitrogen fertilizer, particularly suitable for crops such as rice. For every ton of carbonate acid disodium salt produced, approximately one ton of ammonium chloride is also generated. This approach transforms a traditional cost center—waste treatment—into a profit center through fertilizer production, delivering considerable economic benefits.
In contrast to the ammonia-soda process, which generates large volumes of waste liquid containing calcium chloride that is difficult to manage and highly polluting to soil and water sources, the dual-alkali process achieves “turning waste into treasure.” It eliminates waste discharge at the source and is closely aligned with China’s national policies on environmental protection and sustainable development.
For Western countries, the modernized ammonia-soda process remains an economically viable option:Europe and America possess abundant, easily accessible underground rock salt deposits and salt lakes, which result in very low raw salt costs. Consequently, the urgency to improve salt utilization rates—a key advantage of the dual-alkali process—is much lower than in China.
The ammonia-soda technology was first invented in 1861. Over the years, large Western chemical companies have established extensive and mature production systems for this method. This long-standing industrial presence has led to significant sunk costs, which now deter investment in entirely new production technologies.
Furthermore, differences in agricultural structure also play a role. Western countries, with their large-scale farming systems, predominantly use mainstream nitrogen fertilizers such as urea and ammonium nitrate. As a result, the market for ammonium chloride remains relatively small. In addition, its high chloride content makes it unsuitable for chloride-sensitive crops like tobacco and potatoes. If the dual-alkali process were adopted, the large quantities of by-product ammonium chloride could lack market demand and become a financial burden rather than a source of revenue.
Technical Complexity
The ammonia-soda process is mature and proven, delivering outstanding and consistent product quality. However, it requires large-scale equipment and has relatively high energy consumption.
The dual-alkali (Hou’s) process, in comparison, is technically more complex. While it also yields excellent product quality and can offer superior overall economic benefits, it necessitates a higher initial investment. A critical factor for its success is the market demand and value of its ammonium chloride by-product, which directly impacts its economic viability. Furthermore, the process control of the dual-alkali method is more challenging, as it requires balancing the production and market dynamics of two products: industrial soda and ammonium chloride.
Therefore, when selecting a process, it is essential to consider these operational complexities, market conditions, and by-product valuation.
3.Conclusion and Future Outlook
In summary, the dual-alkali process demonstrates significant advantages in raw material utilization, environmental protection, and overall economic benefits, representing a more advanced production route. In contrast, while the ammonia-soda process is technologically mature, it faces substantial environmental challenges.
It is foreseeable that these two processes will continue to coexist in the long term. Existing ammonia-soda plants can mitigate their environmental impact through technological upgrades—for example, by adopting carbon capture, utilization, and storage (CCUS) technologies. They may also collaborate with external CO₂ emitters (e.g., power plants and steel mills) to use their emissions as production inputs, thereby converting waste into valuable resources and enabling cross-industrial carbon recycling.
The dual-alkali process, on the other hand, directly addresses the critical environmental bottleneck that hinders the ammonia-soda process, turning waste burdens into economic gains. It is thus set to become a necessary pathway for the green and sustainable development of the calcined soda industry, as well as a primary option for new capacity—particularly in Chinese and other Asian markets.



